The Nigeria Centre for Disease Control (NCDC) says that the new coronavirus drug, Dexamethasone, recently announced by British Government, is yet to be approved for use in the treatment of patients with the viral disease in Nigeria.
The British Prime Minister, Boris Johnson, had on Tuesday said UK researchers identified a new cheap drug, which is effective and has reduced COVID-19 deaths risk.
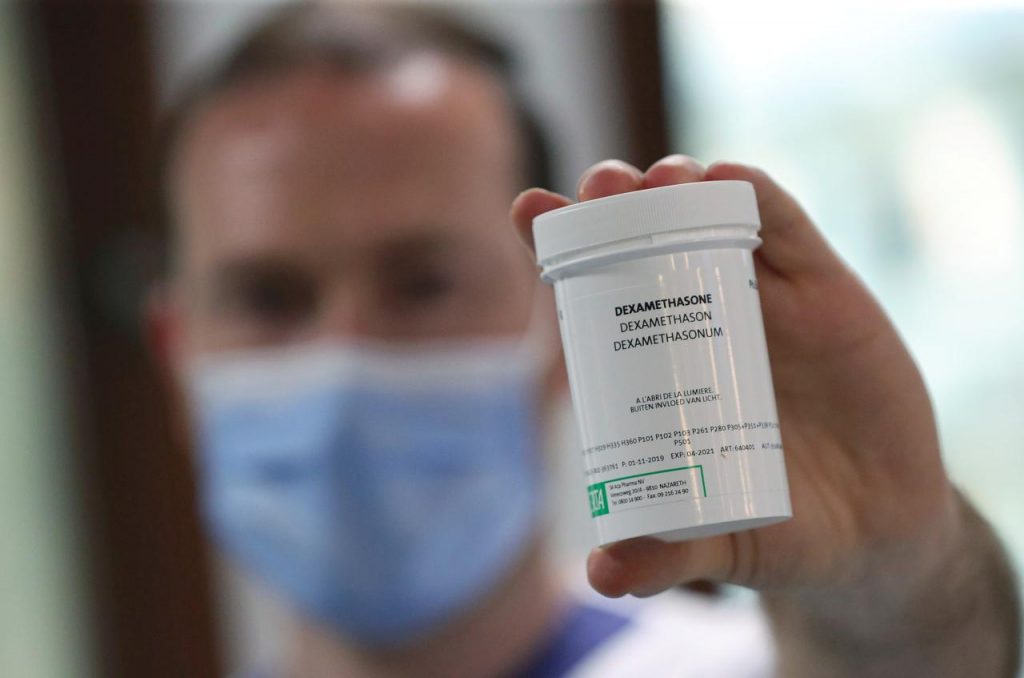
The drug called ‘Dexamethasone’ which was funded by the UK government, has recorded success in its clinical trials after the use of the drug led to a decline in death risks by one-third for COVID-19 patients on ventilators and by one-fifth for patients on oxygen.
Reacting reacting to the report of Dexamethasone showing positive signs, the NCDC said the drug will not be used to treat patients In Nigeria because it has not been approved by the World Health Organization (WHO).
“The Nigeria Centre for Disease Control (NCDC) is aware of recent outcomes from a UK-Government funded clinical trial for COVID-19.
“The results support the use of Dexamethasone as a possible treatment to reduce the risk of death among COVID-19 patients, who require oxygen or mechanical ventilation.
“Please note that the Government of Nigeria has not validated or approved any treatment for COVID-19. In addition, the use of Dexamethasone for COVID-19 treatment has not been validated by WHO.
“We are aware of ongoing clinical trials conducted by scientists in the UK and will work with our sister agencies to evaluate this emerging data on the use of Dexamethasone. We will inform the general public on outcomes following scientific review and validation.”
2/
Please note that the Government of Nigeria has not validated or approved any treatment for COVID-19. In addition, the use of Dexamethasone for COVID-19 treatment has not been validated by @WHO
— NCDC (@NCDCgov) June 16, 2020
Meanwhile, NCDC on Tuesday announced that 490 new cases of coronavirus has been recorded, bringing the total number of infection in the country to 17,148.







![Tinubu Hosts Ghana’s President-Elect, John Mahama In Abuja [Photos] 11 Tinubu Hosts Ghana’s President-Elect, John Mahama In Abuja [Photos]](https://media.kanyidaily.com/2024/12/17161919/tinubu-and-mahama-2-1536x1025-1-768x513-1-150x150.jpg)

![I Don’t Advocate For Beating Children, My Son Breaks TV For Fun When Angry – Bovi [Video] 15 I Don’t Advocate For Beating Children, My Son Breaks TV For Fun When Angry – Bovi [Video]](https://media.kanyidaily.com/2024/12/17144026/Bovi-150x150.jpg)
!["God Delayed My Success For A Purpose" - Tiwa Savage [Video] 17 "God Delayed My Success For A Purpose" - Tiwa Savage [Video]](https://media.kanyidaily.com/2024/12/17140929/Tiwa-Savage-150x150.jpg)

![Nancy Isime Acquires New House To Celebrate Her 33rd Birthday [Photos] 21 Nancy Isime Acquires New House To Celebrate Her 33rd Birthday [Photos]](https://media.kanyidaily.com/2024/12/17123452/Nancy-Isime-150x150.jpg)


